Probiotic Scandal Uncovered: The Shocking Truth About VSL#3 and My Apology
Dear Readers,
For 25 years, I've built my reputation on providing reliable, research-backed health advice you can trust. Today, with deep humility and sincerity, I must share a difficult truth: the probiotic known as VSL#3, which I've repeatedly recommended based on extensive clinical evidence, is not the same formula that's been scientifically validated. This isn't merely a brand mix-up; it's a fundamental difference that matters profoundly for your health. Here's what you need to know immediately, and why it may change everything you thought you knew about probiotics.
I owe you an apology. Despite my commitment to thorough research, I missed crucial information that should have changed my recommendations years ago. There's no excuse for this oversight, and I take full responsibility for not discovering this sooner.
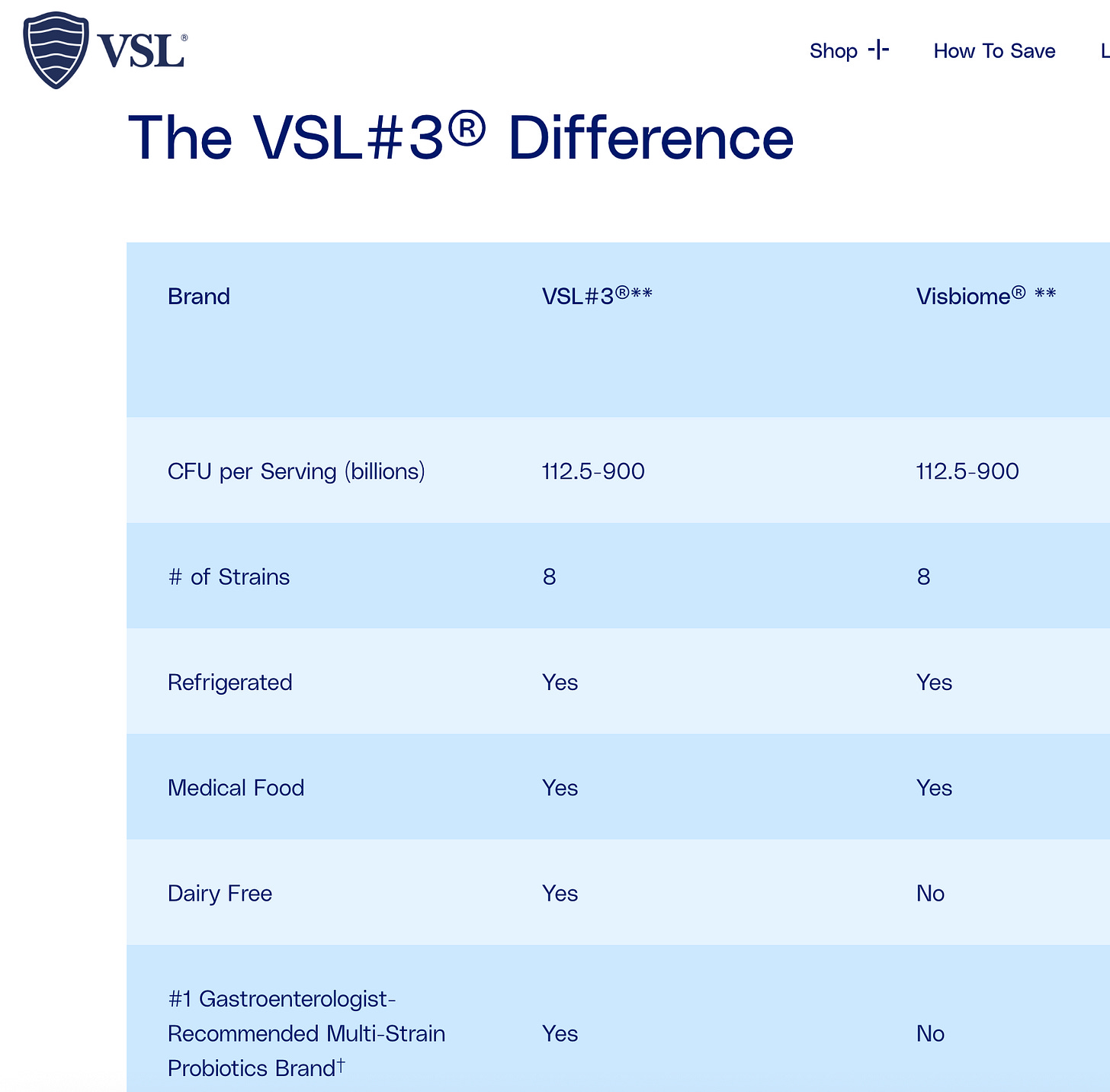
It all began this morning when my wife said that we were almost out of VSL#3 and asked if I could order more. I went to the website to reorder as I always do, and for some reason, the chart below caught my eye.
I wondered what the specific difference was between VSL#3 and Visbiome (they looked very similar), so my squirrelly brain took me on a tangent to find out. As I explored, I felt discomfort spread throughout my body; it was like I was reading a medical conspiracy.
Here's what I got wrong, and what you need to know.
The VSL#3 Story
The Short Version
Suppose you're currently taking VSL#3 based on my recommendations. In that case, you need to know that the product you're buying is NOT the same formulation proven effective in over 100 clinical trials. The original, clinically validated formulation is now only available in the United States and Canada under the brand name Visbiome.
The Full Story
Here's what happened, and what I failed to uncover in my research:
The Original Formulation VSL#3 was initially developed by Professor Claudio De Simone, an Italian researcher who created a specific 8-strain probiotic blend. This formulation, let's call it the "De Simone Formulation," was the subject of extensive clinical research showing remarkable benefits for:
Ulcerative colitis
Pouchitis
IBS
Various inflammatory conditions
I've cited all those studies over the years. They used this original De Simone Formulation.
The Split: In 2016, Professor De Simone had a falling out with the company selling VSL#3 (which had become Alfasigma). He took his original formulation and partnered with a new company, ExeGI. Meanwhile, Alfasigma continued selling a product under the VSL#3 name but with a different formulation.
The Legal Battle: This led to a federal court case where De Simone sued for false advertising. In 2018, a Maryland federal court ruled in De Simone's favor, finding that:
The current VSL#3 is "materially different" from the original formulation
Alfasigma was falsely citing studies of the original formulation as if they applied to their new product
The court awarded nearly $20 million in damages
The Scientific Fallout: The situation became so serious that the Journal of Crohn's and Colitis was forced to issue expressions of concern for 40 articles mentioning VSL#3, warning readers that "the formulation of the currently available VSL#3 may not be the same as the VSL#3 that has been scientifically assessed."
What's Different?
The current VSL#3 sold in the US:
May contain only seven strains instead of the original 8
Uses a synthetic, non-dairy fermentation medium (vs. the original dairy-based medium)
Has not disclosed the specific strain designations
Has NO clinical trials proving equivalence to the original formulation
May have different bacterial counts and ratios
The original De Simone Formulation (now Visbiome) contains these specific strains:
Streptococcus thermophilus DSM 24731
Bifidobacterium breve DSM 24732
Bifidobacterium longum DSM 24736
Bifidobacterium infantis DSM 24737
Lactobacillus paracasei DSM 24733
Lactobacillus plantarum DSM 24730
Lactobacillus acidophilus DSM 24735
Lactobacillus delbrueckii subsp. bulgaricus DSM 24734
Why This Matters
This isn't just about brand names or corporate disputes. The specific strains, their ratios, and how they're cultured can dramatically affect a probiotic's effectiveness. Changing the formulation means you can't assume the clinical benefits transfer. It's like changing the ingredients in a medication and still claiming it works the same way.
My Recommendations Going Forward
If you're taking VSL#3 for a serious condition (IBD, pouchitis, etc.), you should know you're not getting the clinically proven formulation. Consider switching to Visbiome, which contains the original De Simone Formulation.
If VSL#3 is working for you, you don't necessarily need to switch. But understand that your positive experience is anecdotal, not backed by the clinical research on the original formulation.
For new users, I now recommend Visbiome over VSL#3 for anyone seeking the benefits demonstrated in clinical trials.
Always verify that the probiotic you're buying matches the one used in the studies you're relying on.
A Personal Reflection
This discovery has been deeply humbling. It's reminded me that even with decades of experience and a commitment to staying current, we can miss crucial information. The probiotic industry moves fast, with corporate changes and legal battles that don't always make headlines in medical journals.
Going forward, I'm implementing new research protocols to detect these kinds of changes. I'll also review all my previous probiotic recommendations and update them as needed.
I apologize to anyone who purchased VSL#3 based on my recommendations, believing they were getting the clinically studied formulation. While the current VSL#3 may still provide benefits, you deserve accurate information about your purchase.
Thank you for trusting me with your health decisions. I don't take that trust lightly, and I'm committed to earning it daily, including today, when I admit I got something wrong.
Your health journey deserves nothing less than complete transparency and the most accurate information available. I failed to provide that with VSL#3, but I'm committed to improving.
With sincere apologies and renewed commitment to accuracy,
Dr. Dan
P.S. I'll review all my supplement recommendations over the coming weeks to ensure nothing else has slipped through the cracks. If you have questions about any specific products I've recommended, please don't hesitate to reach out.
References
Clinical Research and Strain Information
· Biagi, E., Candela, M., Fairweather-Tait, S., Franceschi, C., & Brigidi, P. (2012). Ageing and gut microbes: Perspectives for health maintenance and longevity. Pharmacological Research, 69(1), 11–20. https://doi.org/10.1016/j.phrs.2012.10.005
· Ciorba, M. A. (2012). A gastroenterologist’s guide to probiotics. Clinical Gastroenterology and Hepatology, 10(9), 960–968. https://doi.org/10.1016/j.cgh.2012.03.024
· Gionchetti, P., Rizzello, F., Helwig, U., Venturi, A., Lammers, K. M., Brigidi, P., ... & Campieri, M. (2003). Prophylaxis of pouchitis onset with probiotic therapy: A double-blind, placebo-controlled trial. Gastroenterology, 124(5), 1202–1209. https://doi.org/10.1016/S0016-5085(03)00194-4
· Tursi, A., Brandimarte, G., Papa, A., Giglio, A., Elisei, W., Giorgetti, G. M., & Di Mario, F. (2010). Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: A double-blind, randomized, placebo-controlled study. American Journal of Gastroenterology, 105(10), 2218–2227. https://doi.org/10.1038/ajg.2010.218
· Visbiome. (n.d.). Strain Information. ExeGi Pharma. Retrieved July 22, 2025, from https://www.visbiome.com/pages/strain-information
Legal Case and Settlement Information
· United States District Court, District of Maryland. (2018). ExeGi Pharma, LLC v. VSL Pharmaceuticals, Inc., et al. Case No. 8:15-cv-01356. Jury Verdict dated November 20, 2018.
· Harvard Law School. (2019, February 4). False advertising verdict against probiotic VSL#3 upheld in federal court. Harvard Law - Petrie-Flom Center. Retrieved from https://billofhealth.wordpress.com/2019/02/04/false-advertising-verdict-against-probiotic-vsl3-upheld-in-federal-court/
· Patexia. (2022, March 28). CAFC affirms judgment in VSL#3 probiotic formulation patent and trademark case. Patexia Legal Insights. Retrieved from https://www.patexia.com/feed/cafc-affirms-judgment-in-vsl-3-probiotic-formulation-patent-and-trademark-case-20220328
Dairy vs. Dairy-Free Probiotic Efficacy
· Marco, M. L., Pavan, S., & Kleerebezem, M. (2006). Towards understanding molecular modes of probiotic action. Current Opinion in Biotechnology, 17(2), 204–210. https://doi.org/10.1016/j.copbio.2006.02.005
· Ranadheera, R. D. C. S., Baines, S. K., & Adams, M. C. (2010). Importance of food in probiotic efficacy. Food Research International, 43(1), 1–7. https://doi.org/10.1016/j.foodres.2009.09.009
· Saad, N., Delattre, C., Urdaci, M., Schmitter, J.-M., & Bressollier, P. (2013). An overview of the last advances in probiotic and prebiotic field. LWT - Food Science and Technology, 50(1), 1–16. https://doi.org/10.1016/j.lwt.2012.05.014
· Sanders, M. E. (2003). Probiotics: Considerations for human health. Nutrition Reviews, 61(3), 91–99. https://doi.org/10.1301/nr.2003.mar.91-99
Additional Resources (Manufacturing, Strain Variation, and Labeling Claims)
· Visbiome. (2024). Frequently Asked Questions. Retrieved July 22, 2025, from https://www.visbiome.com/pages/faqs
· Health Canada. (2019). Monograph: Probiotic supplements. Government of Canada. Retrieved from https://www.canada.ca/en/health-canada/services/drugs-health-products/natural-non-prescription.html
· U.S. FDA. (2023). Labeling of dietary supplements. U.S. Food & Drug Administration. Retrieved from https://www.fda.gov/food/dietary-supplement-products-ingredients/dietary-supplement-labeling-guide




Much respect to anyone who can admit an error and fix it.
Thank you